This is about a serendipity that happened recently for me :P. I had kept a bit of salt water in a glass for 3-4 days and forgotten about it. When I finally noticed it and checked something amazing happened. There were these beautiful salt crystals formed inside it. I wanted to test the formation again so I tried it with a saturated salt solution on a dish for again for 3 days, and bam! the results.

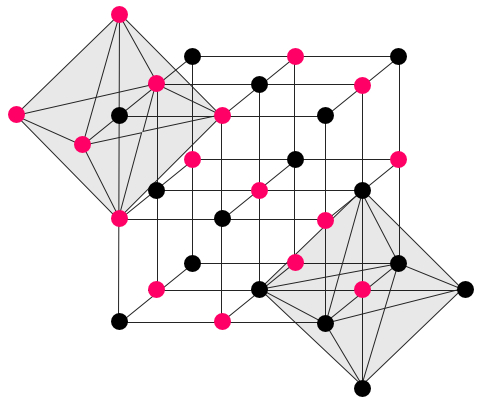
NaCl is one of the fastest growing crystals in nature. It is having cubic crystalline structure with face centered lattice and octahedral geometry.
There was a transparent “X” symbol inside each crystal blocks. This was happening because cube edges grew faster than cube faces, thus forming four pyramid skeletons inside a cube, which appears as “X” symbol from top view.
Crystals are supposed to be formed in cube shape. Since the water level was low, large crystals formed were blocks with square base (height was not equal to base edge), though small crystals followed exact cube shape.
Among all the crystals, 2 of them had octahedron shape.

Figure below can explain better why the NaCl crystals can form in cubic or octahedral shapes:
When I found this, I was like “oh Ya!! I am like the Heisenburg of Breaking Bad” who just did a perfect crystallization ;).